How Gut Microbiome Imbalances can lead to Inflammatory Disorders
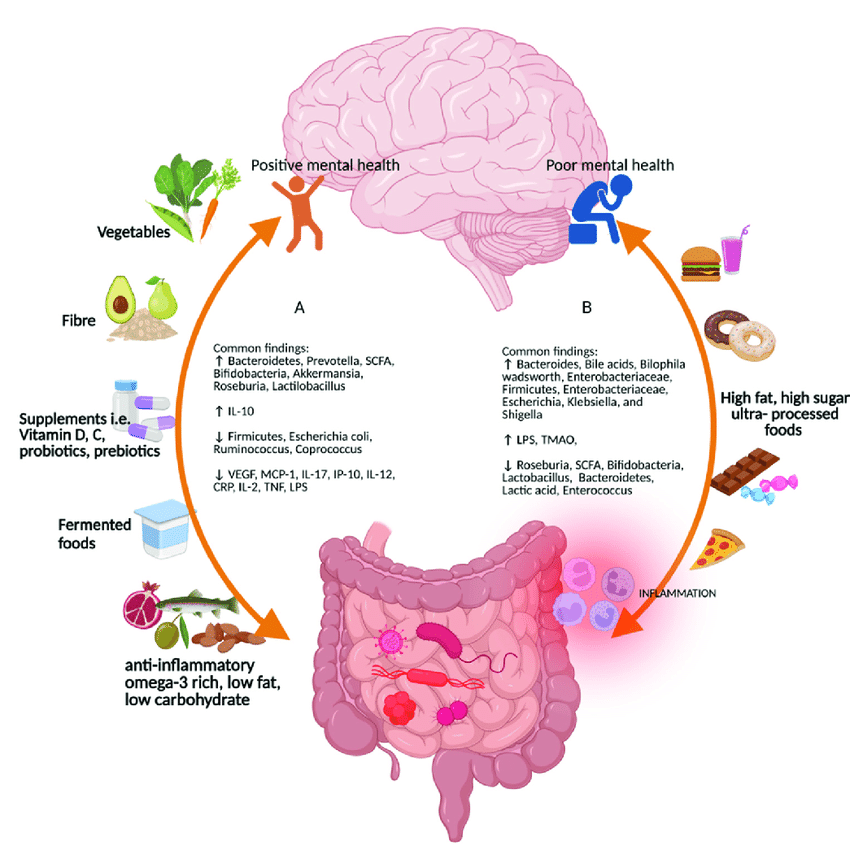
The gut microbiome, also known as the diverse population of bacteria that live in the gastrointestinal system, is an essential component in the process of preserving general health. For the intestines and the rest of the body to operate as they should, it is necessary to maintain a healthy level of what is called eubiosis, or microbial diversity. However, when there is an imbalance in the gut microbiome, a condition known as dysbiosis, it can have a severe impact on the body's health, notably in the form of inflammatory conditions. This can be a significant problem.
The main assault to our immune system by way of the human gut microbiota can be attributed to certain foods. Processed food, added sugar like substances, and low-fiber diets have been linked to gut microbiota and immune function problems. Dysbiosis occurs when helpful bacteria decrease and pro-inflammatory bacteria grow. If it comes in a package or a box with more than one ingredient, it's sustect. These foods can disturb the gut flora and cause immunological responses, leading to chronic inflammation and inflammatory disorders. Chronic inflamation is linked to most diease processes.
Irritable bowel syndrome (IBS) and inflammatory bowel disease (IBD) are both examples of inflammatory illnesses. These conditions are characterized by persistent inflammation in the gastrointestinal tract. It is not entirely clear what causes these conditions, but there is accumulating evidence to suggest that an imbalance in the gut microbiome may play a crucial role in their development.
There are a number of causes that can lead to dysbiosis, including the use of antibiotics, high levels of stress, an unhealthy diet, and chronic illnesses. Antibiotics, for instance, can throw off the delicate balance of the microbiome in the stomach by eradicating helpful microbes and allowing pathogenic ones to flourish unchecked. This overpopulation of harmful microbes can lead to an increase in the synthesis of pro-inflammatory chemicals, which can then enter the bloodstream and travel to other areas of the body, resulting in systemic inflammation.
Another potential consequence of abnormalities in the gut microbiota is the development of a condition known as intestinal permeability or a leaky gut. A condition known as leaky gut happens when the membrane that normally separates the gut from the rest of the body becomes porous. This makes it possible for potentially hazardous microbes and the byproducts they produce to enter the bloodstream. This can cause an immunological response, which can then lead to chronic inflammation, not only in the digestive tract but also throughout the body.
Unbalances in the gut microbiota have been linked to an excessive immune response as another possible cause. The immune system is educated to react correctly to a wide variety of allergens and infections when the stomach is healthy and functioning properly. However, if there are imbalances in the gut microbiome, this can cause the immune system to become hyperactive, which can result in chronic inflammation and an increased risk of inflammatory diseases.
Additionally, certain types of bacteria that are enlarged during dysbiosis are capable of activating the inflammasome, which is a set of proteins that play a vital role in the inflammatory response. This can occur when dysbiosis occurs.
The generation of beneficial bacteria that are responsible for the creation of short chain fatty acids (SCFAs) might also be reduced when dysbiosis is present. SCFAs are extremely important molecules because of their function in controlling the activity of immune cells and preserving the integrity of the gut barrier. Insufficient levels of SCFAs have been linked to increased inflammation as well as an increased risk of inflammatory diseases. Emerging evidence from the HMP suggests that resident microbiota actively shape immune microenvironments within the GI tract. Microbes influence the composition of immune cells, modulate immune cell signaling pathways, and regulate the production of immunomodulatory molecules, such as cytokines and short-chain fatty acids. These immunomodulatory properties of the microbiota contribute to maintaining immune balance and preventing excessive inflammation.
Science geek alert! See the bottom of the page for more information on immunomodulatory molecules.
In addition, there is growing evidence to show that imbalances in the gut microbiome may contribute to the development of autoimmune illnesses such as rheumatoid arthritis, lupus, and multiple sclerosis. Autoimmune disorders are caused when the immune system erroneously assaults healthy cells in the body. Imbalances in the gut microbiota can lead to the activation of immune cells and the creation of pro-inflammatory chemicals, which increases the risk of autoimmune disorders.
The gut microbiome is responsible for a considerable amount of the regulation of the gut-associated lymphoid tissue (GALT), which is an essential component of the immune system. The generation of immune cells as well as the modulation of immunological responses are both under the control of GALT. A decrease in the number of immune cells found in GALT and a reduction in the ability of GALT to respond to allergens and pathogens can both be the result of dysbiosis.
Irritable bowel syndrome (IBS), inflammatory bowel disease (IBD), and autoimmune diseases are only some of the inflammatory conditions that can be caused by an imbalance in the gut flora. These diseases are characterized by persistent inflammation and can have a major influence on the quality of life of the individual who suffers from them. It is possible to build a healthy gut microbiota and enhance overall health by adopting good behaviors such as maintaining a balanced diet, engaging in regular exercise, and finding healthy ways to manage stress. In addition, it may be useful to investigate the possibility of taking probiotic supplements or to consult with a medical practitioner or a qualified dietitian in order to personalize the optimal diet plan. It is essential to keep in mind that despite the fact that having a healthy gut microbiome can assist in lowering the risk of inflammatory disorders, this is in no way a foolproof solution, and further investigation is required to completely comprehend the intricate relationship between gut microbiome imbalances and inflammatory disorders.
Dietary fats have become a biomedical study topic due to obesity. Epidemiological studies show that high-fat diets (HFDs), especially those high in long-chain saturated fatty acids (e.g., Western Diet, National Health Examination survey; NHANES ‘What We Eat in America’ report), cause multi-organ inflammation. Experimental research have verified several illness connections and began to explain disease induction. However, epidemiological studies seem to oversimplify the molecular complexity that depends on dynamic interactions between the host, the particular fatty acid, and the gut microbiota's individualized genetics and variability. Intriguingly, lauric and myristic fatty acid-rich coconut oil may have the opposite impact, improving gut health through unexpected anti-inflammatory and protective pathways.
Science geek area past this point, beware!
I attempted to dig deep into a number of species within the human microbiota and the total numbers.
"It has been estimated that the microbes in our bodies collectively make up to 100 trillion cells, tenfold the number of human cells, and suggested that they encode 100-fold more unique genes than our own genome. The majority of microbes reside in the gut, have a profound influence on human physiology and nutrition, and are crucial for human life. Furthermore, the gut microbes contribute to energy harvest from food, and changes of gut microbiome may be associated with bowel diseases or obesity." From the Journal Nature.
Here are some examples of immunomodulatory molecules produced by the human microbiota:
Short-chain fatty acids (SCFAs): Produced by certain gut bacteria through the fermentation of dietary fiber, SCFAs such as acetate, propionate, and butyrate have been shown to have anti-inflammatory effects and can modulate immune cell function.
Lipopolysaccharide (LPS): LPS is a component of the cell wall of Gram-negative bacteria. While high levels of LPS can trigger an immune response, low levels of LPS from commensal bacteria can help maintain immune homeostasis and promote tolerance.
Polysaccharide A (PSA): PSA is produced by Bacteroides fragilis, a common gut bacterium. It has been shown to promote regulatory T cell (Treg) development and induce anti-inflammatory responses.
Outer membrane vesicles (OMVs): OMVs are small, spherical structures released by various bacteria, including members of the gut microbiota. These vesicles can contain various immunomodulatory components, such as lipids, proteins, and nucleic acids, which can interact with immune cells.
Flagellin: Flagellin is a protein component of bacterial flagella. It can interact with specific pattern recognition receptors in the gut, such as Toll-like receptor 5 (TLR5), and induce immune responses. Flagellin produced by certain gut bacteria has been shown to promote regulatory immune responses.
Bacteriocins: Bacteriocins are antimicrobial peptides produced by certain bacteria. Some bacteriocins have been found to have immunomodulatory effects, influencing immune cell function and regulating the microbiota composition.
Extracellular vesicles: Similar to OMVs, extracellular vesicles are released by various microbes and can contain proteins, lipids, and nucleic acids. These vesicles can interact with immune cells and modulate immune responses.
Indole derivatives: Indole and its derivatives are produced by certain gut bacteria through the breakdown of dietary tryptophan. They can modulate immune cell function and influence intestinal homeostasis.
Secretory IgA (sIgA): Secretory IgA is an antibody produced by plasma cells in the gut mucosa. It plays a crucial role in the maintenance of intestinal immune homeostasis by binding to microbes and antigens, preventing their interaction with immune cells.
Microbial-derived Antigens: Various microbial-derived antigens, such as peptidoglycan, lipoteichoic acid, and bacterial DNA, can interact with pattern recognition receptors on immune cells and modulate immune responses. These antigens can either promote or suppress immune activation depending on the context.